The pleura serves an important role in normal lung function and pleural abnormalities signify important underlying diseases. We explore the use of lung ultrasound to detecting and interpret pleural line pathologies like subpleural consolidations.

SHARE
TABLE OF CONTENTS
On lung ultrasound, the pleura appears as a bright line just underneath the chest wall, and is flanked by dark rib shadows. It consists of an inner layer that lines the lung (visceral pleura) and an outer layer that lines the chest wall, mediastinum and diaphragm (parietal pleura). The two layers are held together by a small amount of pleural fluid. The pleura's primary role is to reduce friction and maintain negative pressure during breathing. Lung ultrasound is much more sensitive than chest-X ray in detecting pleural abnormalities.
A normal pleural line is thin (less than 3 mm), smooth and continuous. In disease, the pleural line may be thickened or irregular. These characteristics can be combined with subpleural lesions and artifacts (such as A lines or B lines) to form a comprehensive differential diagnosis for lung pathology.
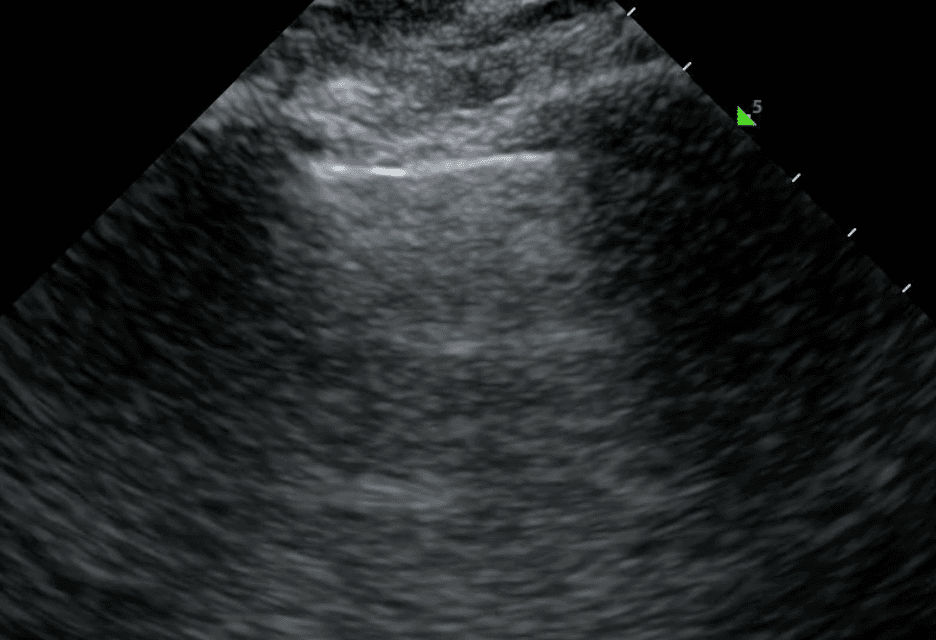
Solid pleural pathologies refer to diseases that affect the pleural line. There is a broad differential diagnosis based on the lung region and specific pleural changes. In the upper regions of the lung, an abnormal pleural line can be associated with acute infection, long-term fibrotic changes, trauma or cancer. In the lower lung regions, pleural changes are usually associated with pleural effusion and atelectasis. Ultimately, to determine the cause of pleural abnormalities, you must consider them in conjunction with the patient’s clinical context and other ultrasound artifacts.
An irregular pleural line describes any disruption to the smooth, continuous appearance of the pleura. This can manifest as jaggedness, unevenness or fragmentation of the pleural line. Pleural thickening refers to a pleura that is thicker than 3mm. In the acute setting, an irregular or thickened pleural line points toward an infectious or inflammatory process such as pneumonia, acute respiratory distress syndrome (ARDS) or, less commonly, malignancy. For a more complete picture, pleural changes should be considered alongside other sonographic features including B lines, lung sliding and consolidation.
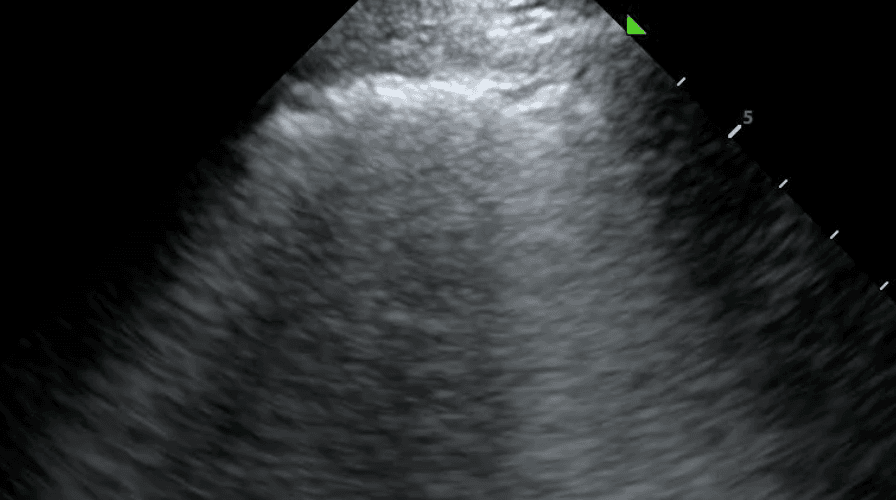
A subpleural consolidation refers to a consolidation underneath the pleural line. While all lung consolidations are technically subpleural, consolidations that extend through the entire lung are referred to as translobar. Compared to translobar consolidations, subpleural consolidations are smaller, tend to occur in the anterior lung and normal tissue can generally be viewed beneath the lesion. They tend to reflect an inflammatory cause, such as pneumonia, infarction from pulmonary embolism and metastatic lesions.
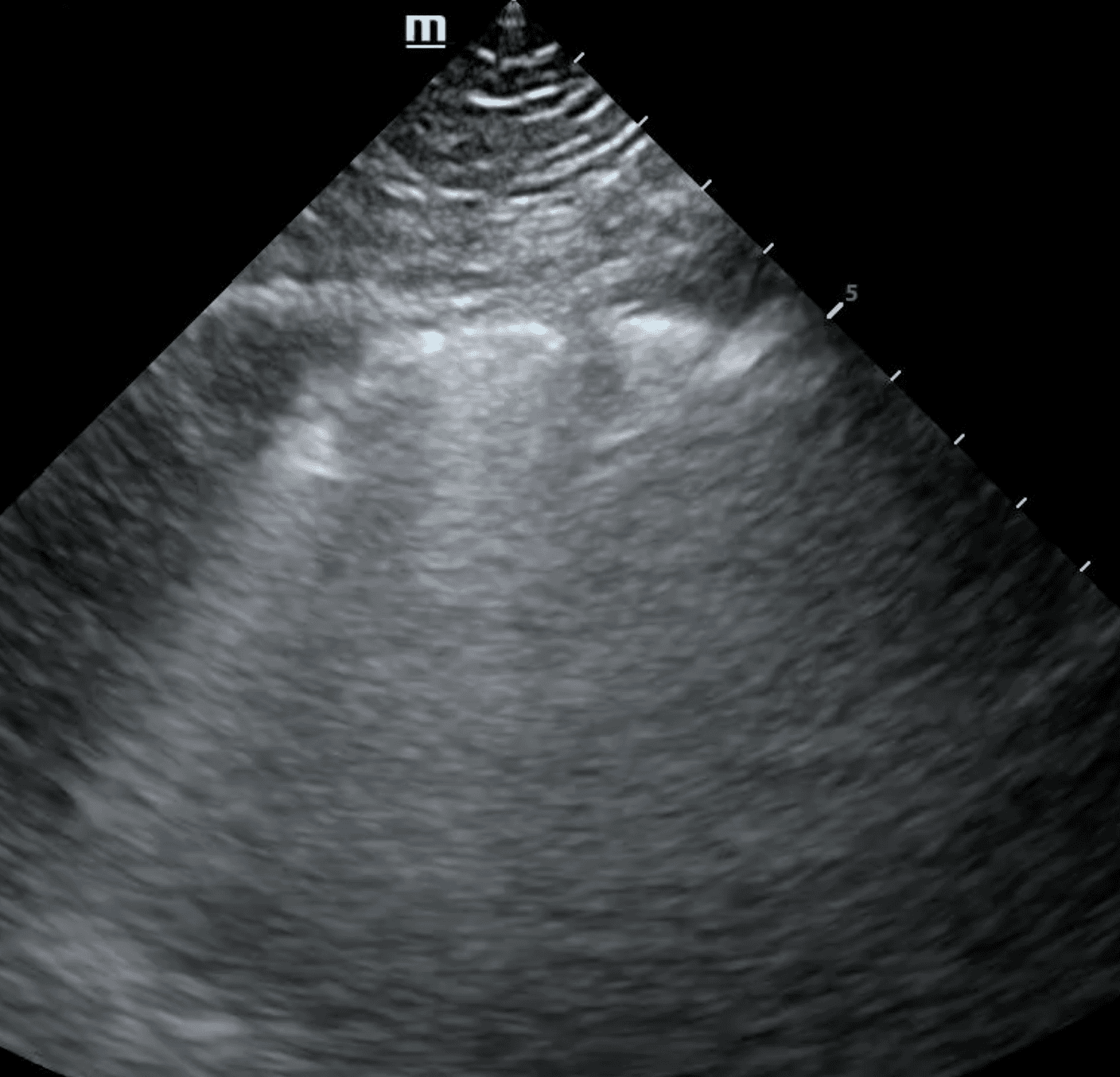
The shred sign, also known as a fractal sign, describes the border between abnormal and normal lung. It separates a subpleural consolidation from underlying normal lung. It has been described as “torn paper” as its irregular boundary contains jagged segments. Visualization of the shred sign helps localize focal consolidations that are likely to be missed on chest X-ray. This allows for early intervention to treat conditions such as pneumonia before they progress.
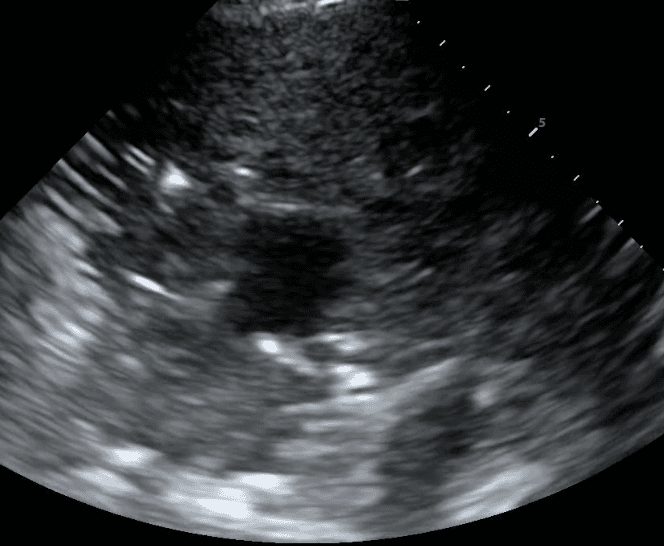
Pleural fibrosis refers to pleural line thickening or pleural plaques in excess of 3 mm. It can be due to acute processes such as bacterial or viral infections, pulmonary embolism, and chemical exposures or from chronic disease, including chronic heart failure, persistent fungal and bacterial infections, autoimmune disorders such as rheumatoid arthritis and lupus, and cancer.
While pleural malignancies are uncommon, a pleural line that has nodularity, is thicker than 10mm, and has associated diaphragm thickness, is strongly indicative of a malignant tumor. There is usually a pleural effusion present alongside the cancer and lung ultrasound can guide a thoracentesis for diagnostic tissue sampling.
While computed tomography (CT) is the current standard of care for cancer identification and staging, lung ultrasound is more sensitive at detecting malignant chest wall invasion. Specifically, ultrasound is able to accurately differentiate small pleural effusions from pleural thickening using colour doppler. This is relevant to current cancer care, as the low cost, absence of radiation, portability and real-time diagnostic information of ultrasound can increase the availability of testing. By improving accessibility through supplementing current diagnostic imaging, patients will be able to get tested and diagnosed sooner, leading to earlier treatment and better outcomes.
Pleural space pathologies comprise any disease affecting the thin, fluid-fill area between the visceral and parietal pleura. These conditions typically involve the excess accumulation of air (pneumothorax), fluid (ie. pleural effusion), blood (hemothorax) or pus (empyema), expanding the space between the pleural layers. This impedes normal lung function and can lead to significant respiratory distress if left untreated.
Lung ultrasound can be invaluable in determining the appropriate management strategy by differentiating these pathologies. For instance, pneumothorax presents with a distinct pattern of absent lung sliding coupled with A lines due to the scattering of sound waves in air (read more here).
In contrast, pleural effusion, hemothorax and empyema may all present with thickening of the pleural line, but can usually be differentiated based on the presence of anechoic fluid, bright echogenic material or septations (read more here). With lung ultrasound, physicians can diagnose and initiate treatment such as chest tube insertion or antibiotics without leaving the bedside.
In conclusion, the pleural line is a primary anatomical landmark in lung ultrasound and its thickness, regularity and movement are crucial for differentiating between many conditions. These can be easily detected by bedside lung ultrasound. Deep Breathe™ is leading a revolution in the use of lung ultrasound that can autonomously detect and characterize the pleural line along with other pleural artifacts. This technology will improve outcomes by allowing healthcare providers to rapidly identify and initiate early treatment for critical lung conditions.
References
Bhoil, R., Ahluwalia, A., Chopra, R., Surya, M., & Bhoil, S. (2021). Signs and lines in lung ultrasound. Journal of Ultrasonography, 21(86), 225–233. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8439137/
Demi, L., Wolfram, F., Klersy, C., De Silvestri, A., Ferretti, V. V., Muller, M., Miller, D., Feletti, F., Wełnicki, M., & Buda, N. (2023). New international guidelines and consensus on the use of lung ultrasound. Journal of Ultrasound in Medicine, 42(2), 309–344. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10086956/
Francisco Neto, M. J., Rahal Junior, A., Vieira, F. A. C., Silva, P. S. D. da, & Funari, M. B. de G. (2016). Advances in lung ultrasound. Einstein (Sao Paulo), 14, 443–448. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5234763/
Husain, L. F., Hagopian, L., Wayman, D., Baker, W. E., & Carmody, K. A. (2012). Sonographic diagnosis of pneumothorax. Journal of Emergencies, Trauma, and Shock, 5(1), 76–81. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3299161/
Kumar, A., & Anand, S. (2020). Lung Decortication. https://www.ncbi.nlm.nih.gov/books/NBK564375/#:~:text=Lung%20decortication%20is%20a%20simple,of%20the%20underlying%20lung%20parenchyma.
Lala, Y. (2016). Lung Ultrasound: Alveolar Consolidation - Shred Sign. WesternSono. https://westernsono.ca/screencasts/lung-ultrasound/alveolar-consolidation-and-shred-sign-2/
Lichtenstein, D. (2017). Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe, 13(2), 100–111. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5467658/
Lichtenstein, D. A. (2014). Lung ultrasound in the critically ill. Annals of Intensive Care, 4, 1–12. https://annalsofintensivecare.springeropen.com/articles/10.1186/2110-5820-4-1
Lichtenstein, D. A. (2015). BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest, 147(6), 1659–1670. https://journal.chestnet.org/article/S0012-3692(15)37223-8/abstract
Messina, G., Bove, M., Natale, G., Di Filippo, V., Opromolla, G., Rainone, A., Leonardi, B., Martone, M., Fiorelli, A., & Vicidomini, G. (2023). Diagnosis of malignant pleural disease: Ultrasound as “a detective probe.” Thoracic Cancer, 14(3), 223–230. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9870740/
von Groote-Bidlingmaier, F., & Koegelenberg, C. F. N. (2012). A practical guide to transthoracic ultrasound. Breathe, 9(2), 133–142. https://breathe.ersjournals.com/content/breathe/9/2/132.full.pdf
SHARE
© 2025, DEEP BREATHE, INC